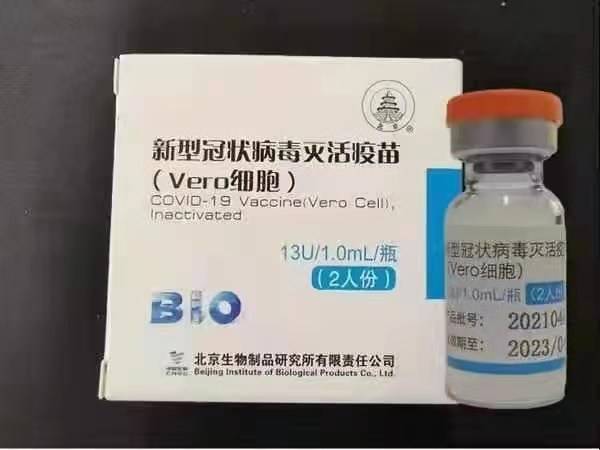
Sinopharm (Beijing): BBIBP-CorV
FASALI 1
1 Gwaji
ChiCTR2000032459
China
FASALI 2
2 Gwaji
NCT04962906
Argentina
ChiCTR2000032459
China
FASALI 3
6 Gwaji
NCT04984408
ChiCTR2000034780
Hadaddiyar Daular Larabawa
NCT04612972
Peru
NCT04510207
Bahrain, Masar, Jordan, Hadaddiyar Daular Larabawa
NCT04560881, BIBP2020003AR
Argentina
NCT04917523
Hadaddiyar Daular Larabawa
Masu amincewa
Lissafin Amfani na gaggawa na WHO Kasashe 59
Angola 、 Argentina 、 Bahrain 、 Bangladesh 、 Belarus 、 Belize 、 Bolivia (Plurinational State of) 、 Brazil 、 Brunei Darussalam Cambodia 、 Kamaru 、 Chadi 、 China 、 Comoros 、 Egypt 、 Equatorial Guinea 、 Gabon 、 Gambia 、 Georgia 、 Guyana 、 Hungary 、 ndonesia 、 Iran (Jamhuriyar Musulunci ta) 、 Iraq 、 Jordan 、 Kyrgyzstan 、 Lao
Lebanon 、 Malaysia 、 Maldives 、 Mauritania 、 auritius 、 Mongolia 、 Montenegro 、 Morocco 、 Mozambique 、 Namibia 、 Nepal 、 Niger 、 North Macedonia 、 Pakistan 、 Paraguay 、 Peru 、 Philippines 、 Jamhuriyar Congo 、 enegal 、 Serbia 、 Seychelles 、 Saliyo Tsibiran 、 Somalia 、 Sri Lanka 、 Thailand 、 Trinidad da Tobago 、 Tunisia 、 Hadaddiyar Daular Larabawa 、 Venezuela (Jamhuriyar Bolivia) 、 Viet Nam 、 Zimbabwe
Sinopharm BBIBP-CorV COVID-19 allurar rigakafin cuta ce da aka yi ta daga ƙwayoyin ƙwayoyin cuta waɗanda ba su da ikon kamuwa da cuta. Sinopharm Holdings da Cibiyar Nazarin Kayan Halittu ta Beijing ne suka haɓaka wannan ɗan takarar rigakafin.
Allurar rigakafin Sinopharm BBIBP-CorV tana aiki ta hanyar barin tsarin garkuwar jiki ya samar da ƙwayoyin rigakafi akan SARS-CoV-2 beta coronavirus. An yi amfani da alluran rigakafin ƙwayoyin cuta shekaru da yawa, kamar allurar rabies da alurar rigakafin hepatitis A. An yi nasarar amfani da wannan fasaha ta ci gaba ga sanannun alluran rigakafi, kamar allurar rabies.
Sinopharm's SARS-CoV-2 iri (nau'in WIV04 da lambar ɗakin karatu MN996528) an ware shi daga mara lafiya a asibitin Jinyintan a Wuhan, China. An yada kwayar cutar a cikin al'adu a cikin layin Vero mai ƙwarewa, kuma ba a kashe ikon ƙwayoyin sel masu cutar da β-propiolactone (1: 4000 vol/vol, 2 zuwa 8 ° C) na awanni 48. Bayan fayyace tarkacewar tantanin halitta da tsaftacewa, an yi rashin aikin β-propiolactone na biyu a ƙarƙashin yanayi iri ɗaya kamar rashin aikin farko. A cewar WHO, an yi allurar rigakafin a kan 0.5 MG na alum kuma an ɗora shi cikin allurai da aka riga aka cika su a cikin 0.5 ml na siliki-buffered saline ba tare da abubuwan kariya ba.
A ranar 31 ga Disamba, 2020, Hukumar Magunguna ta Jiha ta ba da sanarwar amincewa da allurar rigakafin gwaji da Sinopharm ya samar.
A ranar 7 ga Mayu, 2021, Hukumar Lafiya ta Duniya ta ba da sanarwar amincewa da allurar. Jerin amfani da gaggawa na WHO ya baiwa ƙasashe damar hanzarta amincewa da ƙa'idodin su don shigowa da gudanar da allurar COVID-19. Kungiyar kwararrun masu ba da shawara ta WHO kan dabarun rigakafi kuma ta kammala nazarin allurar. Dangane da duk shaidun da ke akwai, WHO ta ba da shawarar allurai biyu na allurar rigakafi, tsakanin sati uku zuwa huɗu, ga manya masu shekaru 18 da haihuwa. Ana kimanta ingancin allurar rigakafin cutar sankara da cututtukan asibiti a kashi 79% ga duk ƙungiyoyin shekaru da aka haɗa.
Ƙungiyar Likitocin Amurka ta buga "Gwajin Asibiti na Rarraba: Tasirin alluran rigakafin SARS-CoV-2 guda biyu akan Ciwon Cutar COVID-19 a cikin Manya" a ranar 26 ga Mayu, 2021, inda suka kammala da cewa "a cikin wannan takaitaccen bincike na gwajin gwaji na asibiti, manya Alluran SARS-CoV-2 marasa aiki guda biyu waɗanda aka gudanar a cikin wannan ƙayyadaddun bincike na gwajin gwaji na asibiti ya rage haɗarin alamun COVID-19, kuma munanan abubuwan da ba a saba gani ba. ” A cikin wannan matakin 3 gwajin bazuwar a cikin manya, ingancin alluran rigakafin ƙwayar cuta guda biyu marasa aiki a cikin alamun COVID-19 alama ce 72.8% da 78.1%, bi da bi. Alluran rigakafin 2 suna da munanan abubuwan da ba su dace ba tare da irin wannan mitar ga ƙungiyar sarrafa alum kawai, kuma yawancinsu ba su da alaƙa da alurar riga kafi. Wani bincike na bincike ya gano cewa alluran rigakafin 2 sun haifar da rigakafin ƙwayoyin rigakafi, wanda yayi daidai da sakamakon gwajin kashi na 1/2.
Kungiyar Aiki ta SAGE ta WHO ta buga bita kan allurar rigakafin COVID-19 na Sinopharm/BBIBP a ranar 10 ga Mayu, 2021. Allurar COVID-19 ta GAVI ta haɗa da na’urar duba allurar rigakafin da ke gaya wa ma’aikatan lafiya ko an adana allurar da kyau kuma ba a fallasa ta ba. zafi fiye da kima. A sakamakon haka, lalacewar, GAVI ta ba da rahoto a ranar 14 ga Mayu, 2021. Labels masu kaifin basira da Kamfanin Zebra Technologies ya ƙera kuma Kamfanin Temptime Corporation ya yi, ya ƙunshi da'irar tare da madaidaicin murabba'i mai launi a tsakiya, wanda aka yi da sinadarai marasa launi waɗanda ba za su iya canza launi ba tsawon lokaci. . Wannan ya zama duhu don bada nuni na gani na tarin zafi. Da zarar an fallasa vial ɗin don zafi fiye da mafi kyawun adadin ajiyarsa, faɗin ya zama duhu fiye da da'irar, yana nuna cewa bai kamata a sake amfani da allurar ba.
Lambar rajista ta kantin magunguna ta BBIBP-CorV COVID-19 lambar rajista: DB15807.